Petroleum is a mixture of hydrocarbons. It is separated into fractions such as petrol, paraffin and diesel. Name two other fractions which are obtained from petroleum.

Important Questions on Cambridge IGCSE Exam Questions from Paper 2
Petroleum is a mixture of hydrocarbons. It is separated into fractions such as petrol, paraffin and diesel. Many of the compounds from petroleum are alkanes. Which two of these are alkanes?
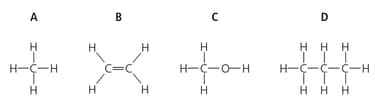
Use words from the list below to complete the following sentence.
ethane ethene hydrogen nitrogen
oxygen reactive unreactive water
Alkanes such as (i)...............are generally (ii).............. but they can be burnt in (iii).............. to form carbon dioxide and (iv)...............
Choose from the list of compounds to answer the question?
calcium carbonate carbon dioxide
methane sodium hydroxide iron(III) oxide
Name the compound which is a transition metal compound.
Choose from the list of compounds to answer the question?
calcium carbonate carbon dioxide
lead(II) bromide methane sodium hydroxide
Name the compound which produces brown fumes at the anode when electrolysed.
Choose from the list of compounds to answer the question?
calcium carbonate carbon dioxide
lead(II) bromide methane sodium hydroxide
Name the compound which is used to manufacture lime.
Choose from the list of compounds to answer the question?
calcium carbonate carbon dioxide
lead(II) bromide methane sodium hydroxide
Name the compound which forms an alkaline solution in water.
