MEDIUM
Earn 100
Phenol is more acidic than alcohol because
(a)Phenol is more soluble in polar solvents
(b)Alcohol does not lose hydrogen atom
(c)Phenoxide ion is stabilised by resonance
(d)Phenoxide ion doesn't exhibit resonance
50% studentsanswered this correctly
Important Questions on Alcohols, Phenols and Ethers
HARD
HARD
What is the product "C" after following reactions -
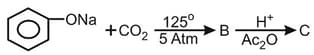
EASY
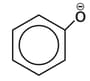 ion ?
ion ?HARD
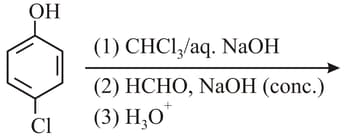
HARD
The major product of the following reaction is:
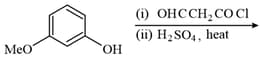
MEDIUM
| Test | Inference | |
|---|---|---|
| Insoluble | ||
| Soluble | ||
| Decolourization |
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
HARD
The increasing order of the values of the following compounds is:
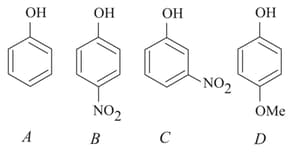
MEDIUM
HARD
EASY
Match the following values.
| Acid | |||
| (a) | Phenol | (i) | |
| (b) | -Nitrophenol | (ii) | |
| (c) | Ethanol | (iii) | |
| (d) | Picric acid | (iv) |
MEDIUM
.
The reactions(s) and respectively are
MEDIUM
the compound is:
MEDIUM
MEDIUM
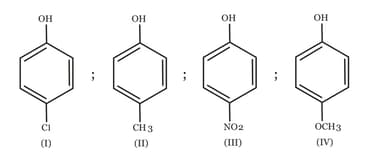
MEDIUM
The functional group which is formed when Phenol is made to react with Chloroform in the presence of dilute Sodium hydroxide

