HARD
Earn 100
Phenol on reaction with a mixture of conc. HNO3 and conc. H2SO4 produces a compound. What is the degree of unsaturation present in the compound and nature of compound ?
(a)5, acidic
(b)7, basic
(c)6, neutral
(d)7, acidic
50% studentsanswered this correctly
Important Questions on Alcohols, Phenols and Ethers
HARD
HARD
What is the product "C" after following reactions -
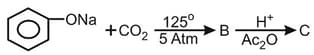
EASY
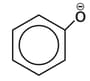 ion ?
ion ?HARD
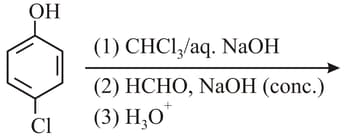
HARD
The major product of the following reaction is:
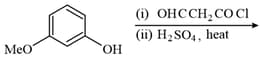
MEDIUM
| Test | Inference | |
|---|---|---|
| Insoluble | ||
| Soluble | ||
| Decolourization |
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
HARD
The increasing order of the values of the following compounds is:
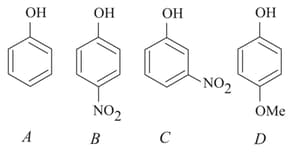
MEDIUM
HARD
EASY
Match the following values.
| Acid | |||
| (a) | Phenol | (i) | |
| (b) | -Nitrophenol | (ii) | |
| (c) | Ethanol | (iii) | |
| (d) | Picric acid | (iv) |
MEDIUM
.
The reactions(s) and respectively are
MEDIUM
the compound is:
MEDIUM
MEDIUM
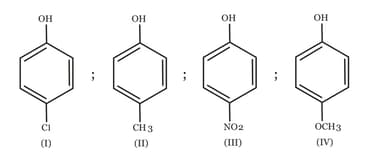
MEDIUM
The functional group which is formed when Phenol is made to react with Chloroform in the presence of dilute Sodium hydroxide

