MEDIUM
Earn 100
Phosphorus is estimated as
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
HARD
1.4 g of an organic compound was digested according to Kjeldahl's method and the ammonia evolved was absorbed in 60 mL of M/10 solution. The excess sulphuric acid required 20 mL of M/10 NaOH solution for neutralization. The percentage of nitrogen in the compound is:
EASY
Reagent, naphthylamine and sulphanilic acid in acetic acid is used for the detection of
EASY
The correct match between item and item is
| Item (Compound) | Item (Reagent) | ||
| Lysine | naphthol | ||
| Furfural | Ninhydrin | ||
| Benzyl alcohol | |||
| Styrene | Ceric ammonium nitrate | ||
EASY
The reddish brown precipitate formed in the Fehling's test for aldehydes (RCHO) is due to the formation of
MEDIUM
When nitrogen and chlorine containing organic compound is reacted with sodium metal, it forms
EASY
Acetic acid reacts with sodium metal at room temperature to produce
HARD
Match List-I with List-II
| List-I | List-II | ||
| Test/Reagents/Observation(s) | Species detected | ||
| (a) | Lassaigne's Test | (i) | Carbon |
| (b) | Cu(II) oxide | (ii) | Sulphur |
| (c) | Silver nitrate | (iii) | , and halogen |
| (d) | The sodium fusion extract gives black precipitate with acetic acid and lead acetate | (iv) | Halogen Specifically |
The correct match is :
EASY
An organic compound ‘A’ is oxidized with followed by boiling with The resultant solution is then treated with ammonium molybdate to yield a yellow percipitate. Based on a above observation, the element present in the given compound is:
MEDIUM
In the detection of nitrogen of an organic compound by Lassaigne's test, a prussian blue colour is obtained. This is due to the formation of which of the following complexes?
MEDIUM
During the fusion of organic compound with sodium metal, nitrogen present in the organic compound is converted into
MEDIUM
In the following reaction
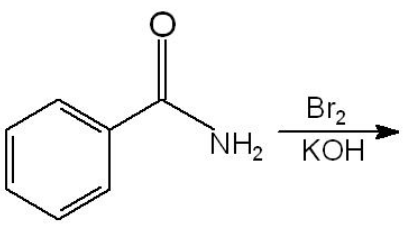
The major product is
EASY
Sodium extract is heated with concentrated before testing for halogens because :
MEDIUM
For the estimation of nitrogen, of an organic compound was digested by the Kjeldahl method and the evolved ammonia was absorbed in of sulphuric acid. The unreacted acid required of sodium hydroxide for complete neutralization. The percentage of nitrogen in the compound is
MEDIUM
In Duma's method for estimation of nitrogen, of an organic compound gave of nitrogen collected at temperature and pressure. If the aqueous tension at is , the percentage of nitrogen in the compound is:
MEDIUM
Which of the following compounds will be suitable for Kjeldahl's method for nitrogen estimation?
MEDIUM
Kjeldahl’s method cannot be used to estimate nitrogen for which of the following compounds?
EASY
In the Kjeldahl's method for estimation of nitrogen present in soil sample, ammonia evolved from of sample neutralized of The percentage of nitrogen in the soil is:
MEDIUM
Which of the following compound is added to the sodium extract before addition of silver nitrate for testing of halogens?
EASY
Which of the following compound will give blood red colour while doing the Lassaigne's test for .
HARD
Match the organic compounds in column - with the Lassaigne's test result in column - appropriately:
| Column – | Column - | ||
| A. | Aniline | i. | Red colour with |
| B. | Benzene sulfonic acid | ii. | Violet color with sodium nitroprusside |
| C. | Thiourea | iii. | Blue color with and acidic solution of |

