MEDIUM
Earn 100
Photoelectric effect supports the quantum nature of light because
(a)There is a minimum frequency of light below which no photo electrons are emitted
(b)The maximum KE of photo electrons depends only on the frequency of light and not on its intensity
(c)Even when the metal surface is faintly illuminated by light of wavelength less than the surface immediately
(d)Electric charge of photo electrons is quantized
50% studentsanswered this correctly
Important Questions on Dual Nature of Matter and Radiation
EASY
( = Planck's constant, = speed of light)
EASY
(Assume mass of electron and Charge of electron )
EASY
MEDIUM
(Mass of electron )
HARD
EASY
MEDIUM
MEDIUM
If the light of wavelength is incident on each of the metals given below, which ones will show photoelectric emission and why?
| Metal | Work Function(eV) |
| Na | 1.92 eV |
| K | 2.15 eV |
| Ca | 3.20 eV |
| Mo | 4.17 eV |
MEDIUM
Given figure shows few data points in a photo-electric effect experiment for a certain metal. The minimum energy for ejection of electrons from its surface is: (Planck's constant )
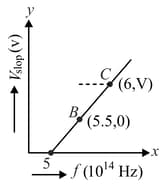
MEDIUM
MEDIUM
MEDIUM
EASY
The work function of a metal is Light of wavelength is incident on this metal. Maximum velocity of photoelectrons is
MEDIUM
EASY
EASY
EASY
EASY
HARD
MEDIUM

