Photons of wavelength fall on a metal surface whose work function is . Assume that each photoelectron inside the metal lattice may come out of the surface orcollide with the lattice before coming out. In each collision with the lattice, it loses of its existing energy. Which of the following can be a kinetic energy of an ejected photoelectron?
Important Questions on Dual Nature of Matter and Radiation
| 0.3 | 2.0 |
| 0.4 | 1.0 |
| 0.5 | 0.4 |
Given that Planck's constant (in units of ) found from such an experiment is :
In a photocell circuit, the stopping potential, is a measure of the maximum kinetic energy of the photoelectrons. The following graph shows experimentally measured values of stopping potential versus frequency, of incident light.
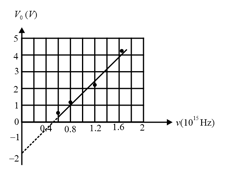
The values of Planck's constant and the work function as determined from the graph are (taking the magnitude of electronic charge to be,),
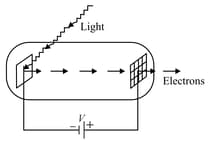
Light of wavelength falls on a cathode plate inside a vacuum tube as shown in the figure. The work function of the cathode surface is and the anode is a wire mesh of conducting material kept at a distance from the cathode. A potential difference is maintained between the electrodes. If the minimum de Broglie wavelength of the electrons passing through the anode is which of the following statement(s) is(are) true ?