HARD
Earn 100
Physical adsorption is an exothermic process .
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Surface Chemistry
EASY
EASY
MEDIUM
EASY
( is the mass of the gas adsorbed per gram of adsorbent)
EASY
MEDIUM
The number of incorrect statement/s from the following is/are
A. Water vapours are adsorbed by anhydrous calcium chloride.
B. There is a decrease in surface energy during adsorption.
C. As the adsorption proceeds, becomes more and more negative.
D. Adsorption is accompanied by decrease in entropy of the system.
EASY
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
Adsorption of the gas increases with:
MEDIUM
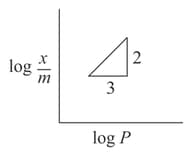
MEDIUM
MEDIUM
(a) becomes less negative as adsorption proceeds.
(b) On a given adsorbent, ammonia is adsorbed more than nitrogen gas.
(c) On adsorption, the residual force acting along the surface of the adsorbent increases
(d) With the increase in temperature, the equilibrium concentration of adsorbate increases.
EASY

