MEDIUM
Earn 100
Pick out the wrong statement(s).
(i) Vapour pressure of a liquid is the measure of the strength of intermolecular attractive forces.
(ii) Surface tension of a liquid acts perpendicular to the surface of the liquid.
(iii) Vapour pressure of all liquids is same at their freezing points.
(iv) Liquids with stronger intermolecular attractive forces are more viscous than those with weaker intermolecular force.
(a)(ii), (iii) and (iv)
(b)(ii) and (iii)
(c)(i), (ii) and (iii)
(d)(iii) only
50% studentsanswered this correctly
Important Questions on States of Matter
HARD
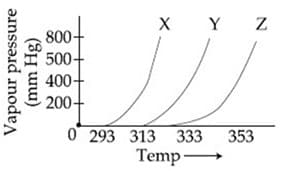
The following inferences are made:
has higher intermolecular interactions compared to
has lower intermolecular interactions compared to
has lower intermolecular interactions compared to
The correct inferences is/are:
MEDIUM
[ Specific heat of water Latent heat of water ]
MEDIUM
HARD
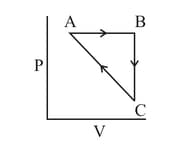
Heat absorbed by the system during process is
MEDIUM
EASY
EASY
HARD

The correct option(s) is (are)
MEDIUM
Based on the given figure, the number of correct statement/s is/are
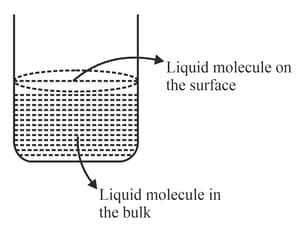
A. Surface tension is the outcome of equal attractive and repulsion forces acting on the liquid molecule in bulk.
B. Surface tension is due to uneven forces acting on the molecules present on the surface.
C. The molecule in the bulk can never come to the liquid surface.
D. The molecules on the surface are responsible for vapour pressure if the system is a closed system.
EASY
(Latent heat of ice is and )
HARD
MEDIUM

HARD
MEDIUM
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
HARD

The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
EASY
MEDIUM
MEDIUM

