EASY
Earn 100
Place a Pyrex glass funnel with its mouth-down in a saucepan full of water, in such a way that the stem tube of the funnel is above the water or pointing upward into the air. Rest the edge of the bottom portion of the funnel on a nail or on a coin, so that water can get under it. Place the pan on a stove and heat it till it begins to boil. Where do the bubbles form first? Why? Can you explain how a natural geyser works using this experience?
Important Questions on Thermal Properties of Matter
EASY
EASY
HARD
MEDIUM
EASY
HARD
Explain the following temperature versus time graph:
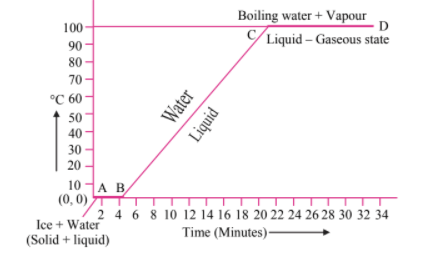
MEDIUM
EASY
MEDIUM
MEDIUM
[ Specific heat of water Latent heat of water ]
EASY
EASY
(Latent heat of ice is and )
EASY
A steam engine intakes of steam at per minute and cools it down to . If latent heat of vaporization of steam is , then the heat rejected by the steam engine per minute is _____
(Given : specific heat capacity of water : )
EASY
Heat required to melt of ice is . A man melts of ice by chewing in one minute. His power is______
HARD
A thermally insulated cubical box of side length and wall thickness containing of ice is closed on all sides. The mass of ice melted in hours is (Thermal conductivity of the material of the box latent heat of ice and ambient temperature )
EASY
HARD
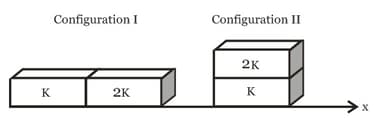
MEDIUM
[Take specific heat of water and latent heat of steam
EASY
EASY

