HARD
Upper Secondary-IGCSE
IMPORTANT
Earn 100
Plot a graph of the melting and boiling point of the halogens against their proton numbers. Join the points for each property together to produce two separate lines on the graph.
Draw a line across the graph at (room temperature) to help you decide which elements are solid, liquid or gas at room temperature and pressure.

Important Questions on Elements and Compounds
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
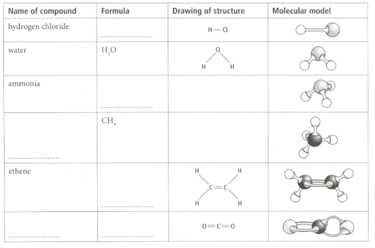
Complete the table by filling in the spaces.
HARD
Upper Secondary-IGCSE
IMPORTANT
Graphite is one of the crystalline forms of carbon. Two of the distinctive properties of graphite are:
- It conducts electricity even though it is a non-metal, and
- It can act as a lubricant even though it has a giant covalent structure.
Give a brief explanation of these properties in light of the structure of graphite.
Graphite as an electrical conductor.
HARD
Upper Secondary-IGCSE
IMPORTANT
Graphite is one of the crystalline forms of carbon. Two of the distinctive properties of graphite are:
- It conducts electricity even though it is a non-metal, and
- It can act as a lubricant even though it has a giant covalent structure.
Give a brief explanation of these properties in light of the structure of graphite.
Graphite are as lubricant.
HARD
Upper Secondary-IGCSE
IMPORTANT
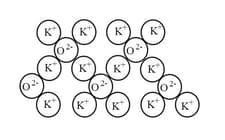
What is the ratio of K+ ions to O2-ions? _____ .
HARD
Upper Secondary-IGCSE
IMPORTANT
The diagram below shows a representation of the structure of an ionic oxide.
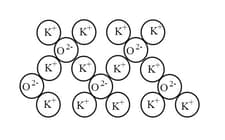
What is the formula of this compound?
