MEDIUM
Earn 100
Polar solvents will dissolve in polar solutes, while non-polar solvents will dissolve in solutes.
100% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
Which of the following solvents are aprotic?
HARD
Identify major product of following sequence?
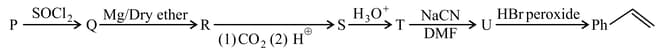
EASY
Suggest the suitable solvent for the reaction given below:
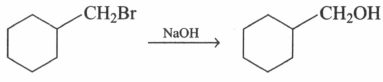
EASY
Which of the following is polar protic solvent?
MEDIUM
Among the following:
I 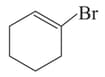
II 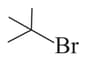
III 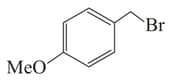
IV 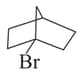
, the compounds which can undergo an reaction in an aqueous solution are:
HARD
Statement - 1: In reactions, use of alcohol as solvent is preferable as compare to and
Statement - 2: Alcohols can dissolve reagent and organic compounds both.
HARD
Give examples of six proton solvents other than water and show how they self-ionize.
EASY
Define solvation energy.
EASY
Solvation energy can be defined as the change in Gibbs energy of a solvent when a solute is dissolved in that solvent.

