MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Polarization means "the distortion of the shape of an anion by an adjacently placed cation". Which of the following statements about polarization is correct
(a)Maximum polarization is brought about by a cation of high charge
(b)Minimum polarization is brought about by a cation of low radius
(c)A large cation is likely to bring about a large degree of polarization
(d)A small anion is likely to undergo a large degree of polarization
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Main/Advance
IMPORTANT
Amongst and , the non-planar species are
EASY
JEE Main/Advance
IMPORTANT
The nature of -bonds in perchlorate ion is :-
HARD
JEE Main/Advance
IMPORTANT
and have the same crystal structure and approximately the same radii. If is the lattice energy of , the approximate lattice energy of is
MEDIUM
JEE Main/Advance
IMPORTANT
The ease of hydrolysis of trichlorides of group elements decreases in the order:-
MEDIUM
JEE Main/Advance
IMPORTANT
Which of the following solid sold have highest value of when heated in closed vessel:-
EASY
JEE Main/Advance
IMPORTANT
Type of bonds between calcium and carbon in are :-
MEDIUM
JEE Main/Advance
IMPORTANT
Ethanol has a higher boiling point than dimethyl ether though they have the same molecular weight. This is due to:
EASY
JEE Main/Advance
IMPORTANT
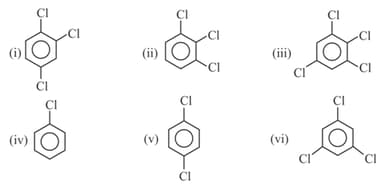
Write order of dipole moment of following compounds :-