Positive charge on the most electronegative atom is unfavorable. Hence, the resonating structure in which charge is present on more electronegative atom(oxygen) is relatively unstable.
In resonance structures, negative charge present on carbon atom is generally less stable than that resonance structure in which charge is on carbon atom(i.e., carbanions are less stable than carbanions generally). Among the following compounds, the strongest acid is?

Important Questions on General Organic Chemistry
In the following compounds,
I 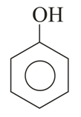
II 
III 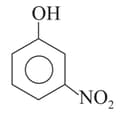
IV 
the order of acidity is:
Most stable carbonium ion is?
The correct order of stability of various resonating structures of methoxybutadiene is:
(i)
(ii)
(iii)
(a)
(b)
(c)
In which of the above pairs of resonance contributors, the structure on the right is an important contributor?
I 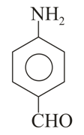
II 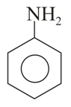
III 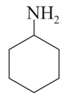
IV 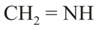
The correct order of bond length in the above compound is:
