EASY
Earn 100
Positive deviation from ideal behaviour takes place because of
(a)Molecular interaction between atoms and PV/nRT > 1
(b)Molecular interaction between atoms and PV/nRT < 1
(c)Finite size of atoms and PV/nRT > 1
(d)Finite size of atoms and PV/nRT < 1
50% studentsanswered this correctly
Important Questions on States of Matter
EASY
For a van der Waals' gas, the term represents some
MEDIUM
EASY
MEDIUM
(i) Their compressibility factor is never equal to unity .
(ii) The deviations from ideal behavior are less at low pressures and high temperatures.
(iii) Intermolecular forces among gas molecules are equal to zero.
(iv) The obey Van der Waals equation, .
EASY
| Gas | ||
|---|---|---|
| A | ||
| B | ||
| C | ||
| D |
and are vander Waals constants. The correct statement about the gases is:
EASY
The unit of the van der Waals gas equation parameter in is:
HARD
HARD
For of gas, the plot of vs is shown below. p is the pressure and is the volume of the gas.
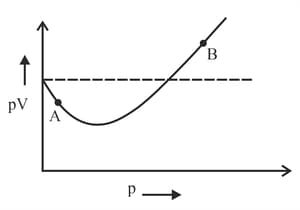
What is the value of compressibility factor at point ?
MEDIUM
HARD
MEDIUM
EASY
EASY
MEDIUM
HARD
HARD
In thermodynamics, the work done is given by . For a system undergoing a particular process, the work done is,
. This equation is applicable to a
HARD
EASY
EASY
HARD
.
This equation reduces to the perfect gas equation, When,

