MEDIUM
Earn 100
Positive deviation from ideal behaviour takes place because of
(a)Molecular interaction between atom and
(b)Molecular interaction between atom and
(c)Finite size of atoms and
(d)Finite size of atoms and
29.41% studentsanswered this correctly
Important Questions on States of Matter: Gases and Liquids
HARD
[Given : " a " and " b " are standard parameters for van der Waals' gas]
MEDIUM
MEDIUM
HARD
EASY
HARD
.
This equation reduces to the perfect gas equation, When,
HARD
MEDIUM
HARD
MEDIUM
EASY
EASY
EASY
EASY
HARD
In thermodynamics, the work done is given by . For a system undergoing a particular process, the work done is,
. This equation is applicable to a
MEDIUM
EASY
MEDIUM
MEDIUM
The variation of the compressibility factorwith pressure for some gases, are shown in the figure below. Identify the gases and .
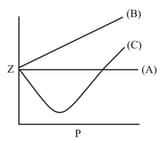
MEDIUM

