EASY
10th CBSE
IMPORTANT
Earn 100
Potassium, bromine and krypton are elements in period of the Periodic Table. In which group of the periodic table Krypton can be found?

Important Questions on Periodic Classification Of Elements
EASY
10th CBSE
IMPORTANT
Potassium, bromine and krypton are elements in period of the Periodic Table.
Bromine exists as a molecule. Draw a 'dot-and-cross' diagram to show the bonding in a molecule of bromine.
EASY
10th CBSE
IMPORTANT
Potassium, bromine and krypton are elements in period of the Periodic Table.
Krypton does not react with either potassium or bromine. Explain the unreactive nature of krypton.
EASY
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
Table given below shows a part of the periodic table.
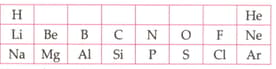
Using this table explain why Li and Na are considered as active metals.
MEDIUM
10th CBSE
IMPORTANT
Table given below shows a part of the periodic table.

Using this table explain why the atomic size of Mg is less than that of Na.
