Precisely mol of helium and mol of neon are placed in a container. Indicate the correct statements about the system.

Important Questions on States of Matter: Gases and Liquids
Which of following statement (s) is(are) true
- The slope of isotherm at the critical point is maximum.
-Larger is the value of , easier is the liquification of gas.
-Vander waal's equation of state is applicable below critical temperature at all pressure.
Consider the following statements: If the Van der Waals parameters of two gases are given as:
| Gas X | ||
| Gas Y |
Then (ii)
(iii):
Select the correct statement.
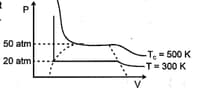
For a real gas, the curve was experimentally plotted, and it had the following appearance. With respect to liquefaction, choose the correct statement:
Consider the following statements:
The coefficient B in the Virial equation of state
(i) is independent of temperature
(ii) is equal to zero at Boyle temperature
(iii) has the dimension of molar volume, Which of the above statements are correct.
Which of the above statements are correct.
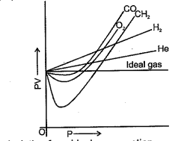
The curve of pressure volume against pressure of the gas at a particular temperature is as shown, according to the graph which of the following is incorrect (in the low pressure region):