EASY
NEET
IMPORTANT
Earn 100
Predict the correct order among the following:
(a)Lone pair - lone pair > lone pair - bond pair > bond pair - bond pair
(b)Lone pair - lone pair > bond pair - bond pair > lone pair - bond pair
(c)Bond pair - bond pair > lone pair - bond pair > lone pair - lone pair
(d)Lone pair - bond pair > bond pair - bond pair > lone pair - lone pair
83.33% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
NEET
IMPORTANT
Which of the following species contains equal number of - and - bonds?
HARD
NEET
IMPORTANT
Which of the following options represents the correct bond order?
MEDIUM
NEET
IMPORTANT
Maximum bond angle at nitrogen is present in which of the following?
MEDIUM
NEET
IMPORTANT
The correct bond order in the following species is:
EASY
NEET
IMPORTANT
The total number of -bond electrons in the following structure is:
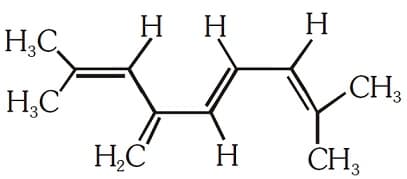
EASY
NEET
IMPORTANT
The formation of the oxide ion, , from oxygen atom requires first an exothermic and then an endothermic step as shown in below:
Thus process of formation of in gas phase is unfavourable though is isoelectronic with neon. It is due to the fact that,
HARD
NEET
IMPORTANT
Decreasing order of stability of and is:
MEDIUM
NEET
IMPORTANT
The variation of the boiling points of the hydrogen halides is in the order . Which of the following option explains the higher boiling point of hydrogen fluoride?
