EASY
AS and A Level
IMPORTANT
Earn 100
Predict the electrolysis products at the anode and cathode when molten zinc oxide.

Important Questions on Electrochemistry
EASY
AS and A Level
IMPORTANT
A concentrated aqueous solution of hydrochloric acid is electrolysed. Write half-equation to show the reaction at the cathode.
EASY
AS and A Level
IMPORTANT
A concentrated aqueous solution of hydrochloric acid is electrolysed. Write half-equation to show the reaction at the anode.
EASY
AS and A Level
IMPORTANT
A very dilute solution of hydrochloric acid is electrolysed. What substance or substances are formed at the anode? Explain your answer.
EASY
AS and A Level
IMPORTANT
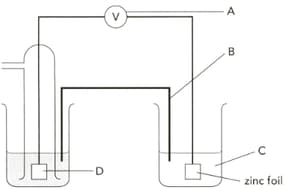
The diagram shows an electrochemical cell designed to find the standard electrode potential of zinc. Name the apparatus labelled "A" and give a characteristic it should have.
EASY
AS and A Level
IMPORTANT
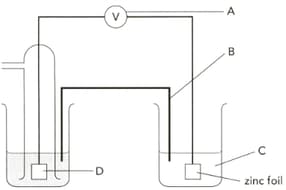
The diagram shows an electrochemical cell designed to find the standard electrode potential of zinc. Name the part "B" and give its two functions.
EASY
AS and A Level
IMPORTANT
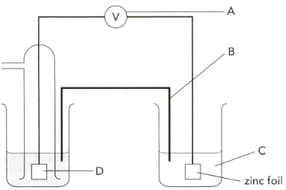
The diagram shows an electrochemical cell designed to find the standard electrode potential of zinc. Describe how part "B" can be prepared?
EASY
AS and A Level
IMPORTANT
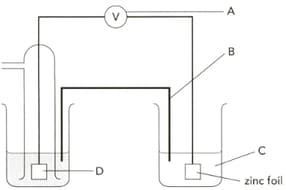
The diagram shows an electrochemical cell designed to find the standard electrode potential of zinc. Name"C" .
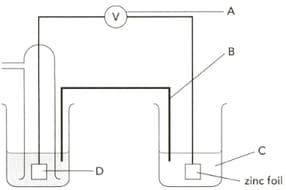
EASY
AS and A Level
IMPORTANT
The diagram shows an electrochemical cell designed to find the standard electrode potential of zinc. Name part "D" and give its two functions.