EASY
Earn 100
Pressure in a bulb dropped from 2000 to 1500 mm in 47 minute when the contained oxygen leaked through a small hole. The bulb was then completely evacuated. A mixture of oxygen and another gas of molecular weight 79 in molar ratio 1:1 at a total pressure of 4000 mm was introduced. Find the molar ratio of two gases remaining in the bulb after a period of 74 minute.
(a)1.236
(b)0.214
(c)1.520
(d)2.587
50% studentsanswered this correctly
Important Questions on States of Matter: Gases and Liquids
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
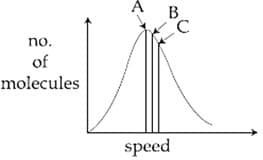
Root mean square speed most proable speed Average speed
HARD
EASY
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
EASY

