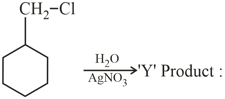EASY
JEE Main/Advance
IMPORTANT
Earn 100
Rate of electrophilic addition on isobutylene is significantly higher than cis or transbutene chiefly due to-
(A)
(B)
(C)
(D)
(a)Lesser stability of 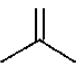 (isobutylene) in comparison to
(isobutylene) in comparison to 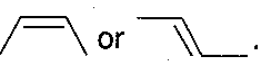
 (isobutylene) in comparison to
(isobutylene) in comparison to 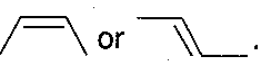
(b)Higher dipole moment of 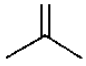 in comparison to cis or transbutene.
in comparison to cis or transbutene.
 in comparison to cis or transbutene.
in comparison to cis or transbutene.(c)Better stabilization of positive charge acquired during formation of bromonium ion intermediate by Me-groups
(d)High angle strain in the molecule
89.47% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
JEE Main/Advance
IMPORTANT
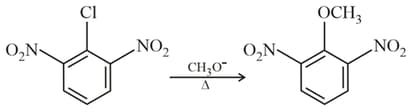
The reaction most likely occurs by which of the following mechanism ?
HARD
JEE Main/Advance
IMPORTANT
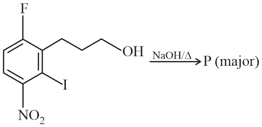
The product is
MEDIUM
JEE Main/Advance
IMPORTANT
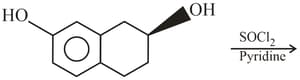
The product is :
MEDIUM
JEE Main/Advance
IMPORTANT
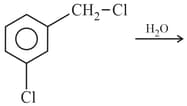
The product is :
HARD
JEE Main/Advance
IMPORTANT
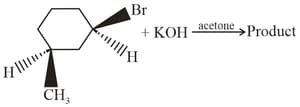
The product formed in the reaction is
MEDIUM
JEE Main/Advance
IMPORTANT
The increasing order of reactivity of the following isomeric halides with ( Alcohol) is :
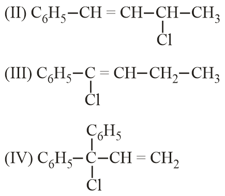
MEDIUM
JEE Main/Advance
IMPORTANT
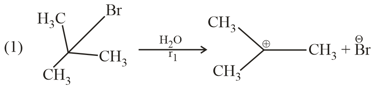
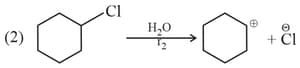
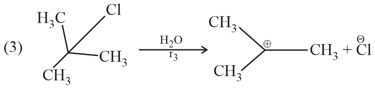
The rates and are in the order :
MEDIUM
JEE Main/Advance
IMPORTANT