EASY
Earn 100
Rate of reaction depends upon
(a)Temperature
(b)Catalyst
(c)Concentration
(d)All of these
100% studentsanswered this correctly
Important Questions on Chemical Kinetics
EASY
EASY
MEDIUM
MEDIUM
Adding surfactants in non polar solvent, the micelles structure will look like
(a)
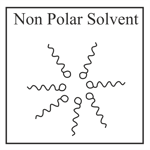
(b)
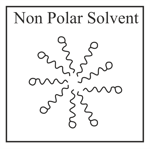
(c)
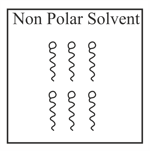
(d)
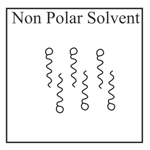
EASY
MEDIUM
The variation of the rate of an enzyme catalyzed reaction with substrate concentration is correctly represented by graph
(a)
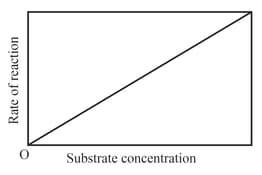
(b)
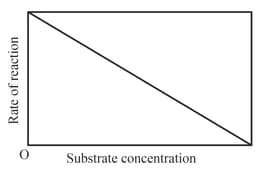
(c)
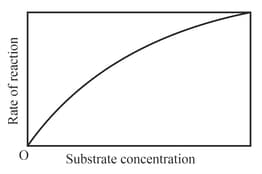
(d)
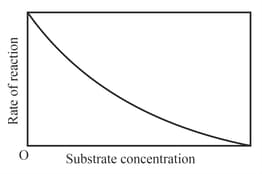
EASY
(a) In which test tube does the reaction proceed faster?
(b) Give reason.
(c) Give an instance in daily life, where such condition is made use.
MEDIUM
HARD
(i) time, (ii) concentration of reactants, (iii) temperature, and (iv) catalyst
MEDIUM
HARD
EASY
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

EASY
HARD
The activation energy of the backward reaction exceeds that of the forward reaction by (in ). If the pre-exponential factor of the forward reaction is times that of the reverse reaction, the absolute value of for the reaction at is ____.
(Given; and is the Gibbs energy)
MEDIUM
A sample of milk splits after at and after at when the population of Iactobacillus acidophilus in it doubles. The activation energy (in ) for this process is closest to ______________.
HARD
EASY
MEDIUM
MEDIUM

