Ravi's science teacher discussed the distinction between physical and chemical change in the classroom. At night, when Ravi was in his house, there was a power cut and his mother asked him to bring a candle. Ravi brought a candle, and his mother ignited it. He saw some changes occurring in the candle, but he could not identify the type of change.
Answer the following question:
When a candle burns, both physical and chemical changes take place. Explain.



Important Questions on Physical and Chemical Changes
A teacher is running an experiment. The demonstration piques the interest of the class. Students were told by the teacher to take a step back from her. She used a flame to burn a ribbon made of magnesium. The ribbon instantly burned up and emitted a brilliant white light colour. The magnesium ribbon became ash after few seconds.
What kind of change is it? Is it exothermic or endothermic?
Identify the chemical composition of the ash produced.

Silver is a soft, white, lustrous transition metal that has long been valued as a precious metal. Silver metal is used in many bullion coins, jewellery, puja articles, show articles, etc.
 .
. 
Rahul's mother had bought some silver spoons a year ago, and today when she opened the box for using them, she found that reddish-brown spots have developed on the spoons. Do you think that the spoon was actually made of silver? Give a simple explanation.


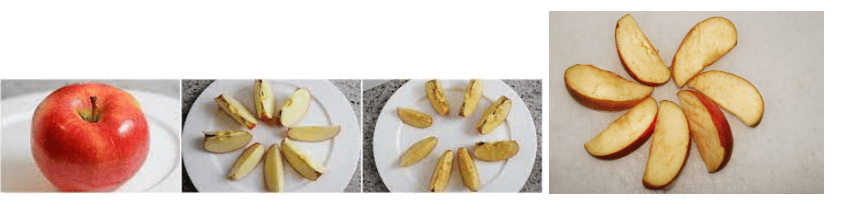
Reema was studying with her friend Ridhima at her home. Reema's mom cut the apple and gave them to eat. They both ate one-one slice of apple and again started doing homework.

After some time they noticed that the remaining slices of apples turned brownish. Why do the cut surfaces of fruits and vegetables turn brown?
When a thread is dipped in a hot copper sulphate solution, blue coloured crystals start to deposit over the thread as the solution becomes cold.

The teacher explained the above activity to her students and described the process as crystallisation. Can you help the students in concluding the nature of this process (physical / chemical) by which crystals are obtained from copper sulphate solution?
Sylvi bought a beautiful statue made of iron. As she has recently learnt in her school that iron gets corroded easily, she went to an ironsmith and asked him to help her in preventing the statue from being corroded. The ironsmith then took the statue and dipped it in some molten liquid. On taking out the statues from the liquid container, the statues now appeared silvery-white, and the ironsmith assured that she should now not worry about the corrosion.
(a) Identify the liquid used by ironsmith.
(b) How does this layer protect the iron statues from corrosion?
When a grey-coloured object made of metal A is left exposed to damp air for a considerable time, it gets covered with a red-brown flaky coating according to a process called B. This red-brown flaky coating eats up the whole object gradually.
It is said that the presence of C and D is necessary for the process B to take place. If this object is galvanised by metal E, then process B gets avoided.

1. Name the metal A from which the object is made.
2. Name the process B and also name the factors C and D which are required for process B to take place.
3. Identify the metal E and describe some other ways by which process B can be prevented.
Ranbir has been invited to his friend Manu's birthday. On his way to the party, Ranbir refilled his bike with petrol at a fuel station. As Ranbir reached the party venue, Manu's mother asked to bring the cake. The cake was lit with different coloured candles. Ranbir congratulated and hugged Manu, and had a great time at the party with his other friends and finally returned home.

From the above understanding answer the following questions:
(a) Burning of petrol in Ranbir's vehicle is a physical change or chemical change? Explain your answer.
(b) Is burning of candle a chemical change?
Ramu once visited the coastal region of Gujarat with his family. There he saw huge ponds where seawater was kept. He asked his father for the reason behind this storage, to which his father said that this storage of seawater in ponds is for extracting salt from seawater. His father also told him that seawater is rich in sodium chloride.
In these ponds, seawater is gathered, evaporated with the help of energy from the sun, and salts are subsequently produced. The crystallisation technique is superior to this process, he said in addition.
a) Why is crystallisation considered a much better technique than evaporation to separate a solid from its solution?
b) Is crystallisation a reversible change?

