Reaction of metal carbonate and bicarbonate with dilute acid to produce gas.
..

Important Questions on Occurence of Carbon and its Compounds in Nature
Production of gas by heating metal carbonates or bicarbonates
Production of gas by heating metal carbonates or bicarbonates
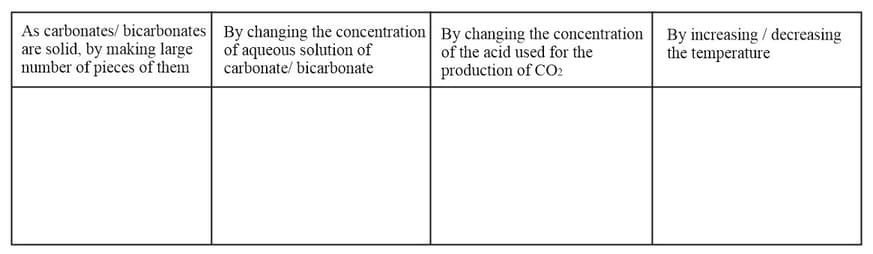
How can the following conditions influence the production of carbon dioxide?
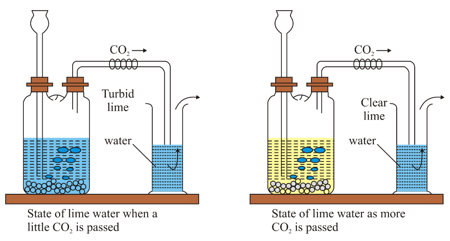
| Experiment | Observation | Why does this happen |
|
1. Take some clear lime water [ solution] in a glass container as shown in the figure. Pass from a Wolf bottle to the lime water. 2. More is passed through the turbid lime water. |
1. Appearance of the lime water. _____ 2. Appearance of the lime water. _____ |
1. Explain after completing the equation. 2. Soluble calcium bicarbonate is formed from insoluble calcium carbonate.
|
For a whitewash at house, quicklime is soaked in water. After two or three days when the slaked lime settles down, solid flakes float over the clear lime water. From the experiment, it can be seen that,
(i) The flakes are of calcium carbonate . How is it formed?
(ii) Some flakes in a test tube and dilute is added to it. What do you see? Write down with equation.
(iii) Some clear lime water is a taken in a test tube and using a straw some air is blown from your mouth through the solution. You will see that the solution is turning turbid with formation of a white precipitate. If the exhaled air contains , then what do you think the white precipitate is made up of? Write down the equation for the reaction that is taking place here.
Complete the following reaction and explain.
Complete the following reaction and explain.
