Read the given passage and fill in the blanks by choosing an appropriate option.
When a limited quantity of water is sprinkled over a dirty white stone called ___(i)___ an ___(ii)___ reaction takes place. After some time, the stone-like substance changes into a white amorphous powder called ___(iii)___ which is used for ___(IV)___.
(i)
(ii)
(iii)
(iv)
(A)
Plaster of Paris
Exo-thermic
Quick lime
Medical purposes
(B)
Quick lime
Exo-thermic
Slaked lime
Whitewashing
(C)
Sodium bicarbonate
Endo-thermic
Sodium carbonate
Baking
(D)
Calcium carbonate
Endo-thermic
Calcium oxide
Whitewashing

Important Questions on Chemical Reactions and Equations
Four test tubes were taken and marked respectively. ml of an aqueous solution of aluminium sulphate was filled in each test tube. A piece of metal zinc was placed in a test tube , iron in a test tube , copper in a test tube and aluminium in a test tube . Mark the correct change in colour in the four test tubes.
| 1 | 2 | 3 | 4 | |
| (A) | Colourless | Green | Blue | Colourless |
| (B) | Light green | Green | Blue | Pale yellow |
| (C) | Light blue | Colourless | Colourless | Light blue |
| (D) | Colourless | Colourless | Colourless | Colourless |
Study the given table carefully.
| S,No. | Metal | Reaction with dilute |
| 1. | P | No change |
| 2. | Q | The temperature of reaction mixture rises |
| 3. | R | Reaction is explosive |
| 4. | S | Some gas bubbles are seen |
Metal P, Q, R and S could be respectively.
Read the given statements and mark the correct option.
Statement 1: When a mixture of hydrogen and chlorine is placed in sunlight, hydrogen chloride is formed.
Statement 2: It is an example of a displacement reaction.
Which of the following reactions are exothermic in nature?
(i) Evaporation of water
(ii) Dissolution of sodium hydroxide in water
(iii) Dilution of sulphuric acid
(iv) Dissolution of ammonium chloride in water
(v) Combustion of methane gas
Marble chips or calcium carbonate react with hydrochloric acid as:
The reaction is carried out twice and the following graphs were obtained:

Which of the following statements is incorrect?
Four students P, Q, R and S noted the initial colour of the solutions kept in beakers I, II, III and IV. After inserting zinc rods in each solution and leaving them undisturbed for two hours, the colour of each solution was again noted in the form of the table given below:
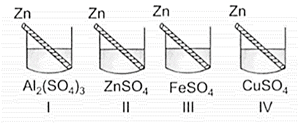
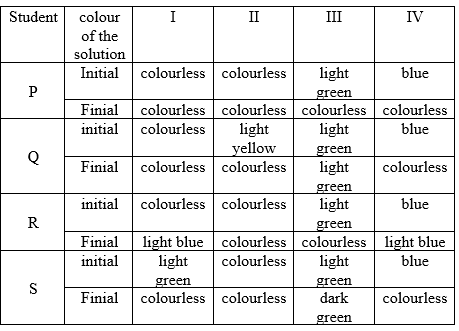
Which student noted the colour change in all the four beakers correctly?
Observe the given figure carefully. Which of the following observation(s) is/are correct?
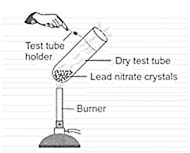
I. A double decomposition reaction takes place.
II. Brown fumes of are evolved.
III. Red residue is left behind in the test tube.
Rupali, a class student has set up the apparatus as shown in the figures.
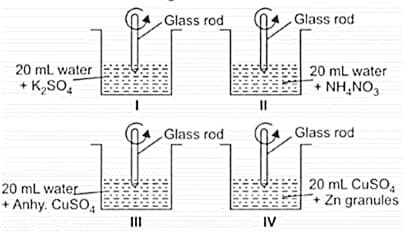
Which of the following observations is correct?
