EASY
Earn 100
Resolution of racemic mixture is not used to separate the isomers present in racemic mixture.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Organic Chemistry: Some Basic Principles and Techniques
HARD
For the given compound X, the total number of optically active stereoisomers is ____.
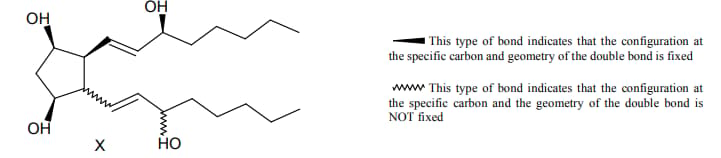
EASY
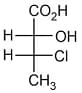 is:
is:MEDIUM
HARD
The Fischer projection of -erythrose is shown below.
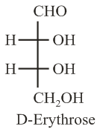
-Erythrose and its isomers are listed as and in Column I. Choose the correct relationship of and with -erythrose from Column II.
| Column - I | Column -II | ||
| P. | 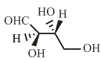 |
1. | Diastereomer |
| Q. | 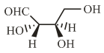 |
2. | Identical |
| R. | 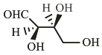 |
3. | Enantiomer |
| S. | 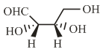 |
HARD
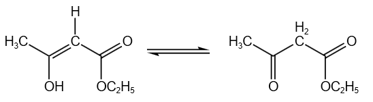
HARD
EASY
MEDIUM
MEDIUM
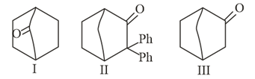
MEDIUM
MEDIUM
EASY
EASY
HARD
MEDIUM
Which of the following carbonyl compounds will exhibit enolization?
(i) 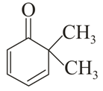
(ii)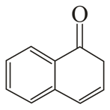
(iii) 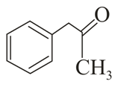
(iv) 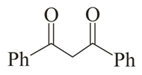
(v) 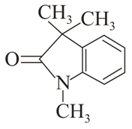
MEDIUM
The absolute configurations of the following compounds 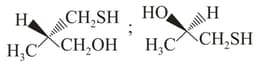 respectively, are
respectively, are
EASY
The correct statement about the following compounds
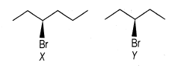 is
is
EASY
MEDIUM
MEDIUM

