EASY
JEE Main/Advance
IMPORTANT
Earn 100
Resonance hybrid of nitrate ion is :-
(a)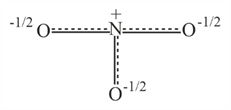

(b)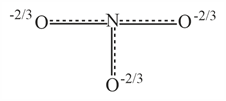

(c)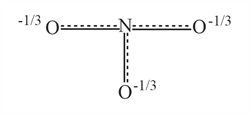

(d)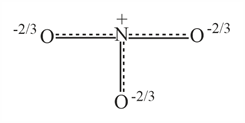

50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
JEE Main/Advance
IMPORTANT
The correct order of bond angle (smallest first) in and is :-
MEDIUM
JEE Main/Advance
IMPORTANT
Nodal planes of bonds in benzene are located in:
MEDIUM
JEE Main/Advance
IMPORTANT
Which of the following has fractional bond order?
MEDIUM
JEE Main/Advance
IMPORTANT
Which is correct statement?
As the -character of a hybrid orbital decreases ______.
(I) The bond angle decreases
(II) The bond strength increases
(III) The bond length increases
(IV) The size of orbital increases
MEDIUM
JEE Main/Advance
IMPORTANT
Which of the following compounds have the same no. of lone pairs with their central atom :-
(IV) Triple methylene
MEDIUM
JEE Main/Advance
IMPORTANT
Select pair of compounds in which both have different hybridization but have same molecular geometry :
MEDIUM
JEE Main/Advance
IMPORTANT
The states of hybridization of boron and oxygen atoms in boric acid are respectively
MEDIUM
JEE Main/Advance
IMPORTANT
Which of the following option w.r.t. increasing bond order is correct ?
