EASY
Earn 100
Rutherford's alpha rays scattering experiment eventually led to the conclusion that
(a)Mass and energy are related
(b)Electrons occupy empty space around the nucleus
(c)Neutrons are buried deep in the nucleus
(d)The point of impact with matter can be precisely determined
75.47% studentsanswered this correctly
Important Questions on Structure of Atom
EASY
EASY
EASY
EASY
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
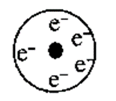
Write the name of particle present at the center of electrons in represented Rutherford's model. Write one characteristic of this model.
EASY
MEDIUM
EASY
EASY
MEDIUM
EASY
Select the correct statements from the following :
A. Atoms of all elements are composed of two fundamental particles.
B. The mass of the electron is .
C. All the isotopes of a given element show same chemical properties.
D. Protons and electrons are collectively known as nucleons.
E. Dalton's atomic theory, regarded the atom as an ultimate particle of matter.
Choose the correct answer from the options given below:
MEDIUM
Write the name of the particle present in the sphere of represented Thomson atomic model. Explain the model with one example.

MEDIUM
EASY

