HARD
Earn 100
STATEMENT - 1: The Arrhenius equation explains the temperature dependence of rate of a chemical reaction.
STATEMENT-2: Plots of log k versus 1/T are linear and the energy of activation is obtained from such plots.
(a)STATEMENT-1 is True, STATEMENT-2 is True, STATEMENT-2 is correct explanation of STATEMENT-1
(b)STATEMENT-1 is True, STATEMENT-2 is True, STATEMENT-2 is NOT correct explanation of STATEMENT-1
(c)STATEMENT-1 is True, STATEMENT-2 is False
(d)STATEMENT-1 is False, STATEMENT-2 is True
38.46% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
(Assume Activation energy and pre-exponential factor are independent of temperature; )
MEDIUM
MEDIUM
EASY
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)
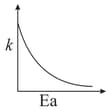
(II)
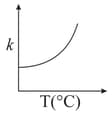
MEDIUM
The Arrhenius plots of two reactions, I and II are shown graphically-
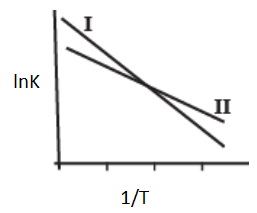
The graph suggests that-
EASY
HARD
It was found that the is decreased by in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre-exponential factor is same)
EASY
EASY
MEDIUM
EASY
MEDIUM
Identify the incorrect statement.
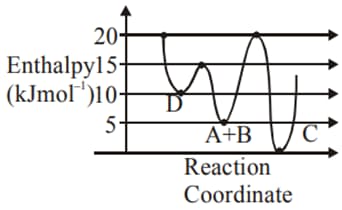
MEDIUM
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
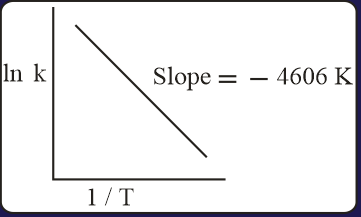
MEDIUM
MEDIUM
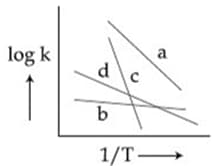
MEDIUM
MEDIUM
MEDIUM
[Gas constant, ]
EASY

