EASY
Earn 100
Scuba divers when go in deep sea face a very painful situation known as "bends", the formation of bubbles around the joints due to decrease in solubility of as a result of reduced pressure, when the diver rises up towards sea surface. This situation is avoided by-
(a)Taking only pure in breathing cylinders.
(b)Replacing by , which is more soluble than .
(c)Replacing by , which is less soluble then .
(d)This situation can not be avoided and therefore pain killers are consumed.
50% studentsanswered this correctly
Important Questions on Solutions
MEDIUM
EASY
EASY
The oxygen dissolved in water exerts a partial pressure of in the vapour above water. The molar solubility of oxygen in water is ______
(Round off to the Nearest Integer).
[Given : Henry's law constant for Density of water with dissolved oxygen]
EASY
MEDIUM
EASY
gas is bubbled through water during a soft drink manufacturing process at . If exerts a partial pressure of then of would dissolve in of water. The value of is _______. (Nearest integer)
(Henry's law constant for at is )
MEDIUM
EASY
MEDIUM
MEDIUM
Henry's constant (in kbar) for four gases and in water at is given below :
(density of water at ) This table implies that :
EASY
HARD
MEDIUM
MEDIUM

The gas with the highest value of Henry's law constant is
EASY
EASY
Aquatic animals are more comfortable in cold water than in warm water.
EASY
From the graph, the value of Henry's constant for the solubility of gas in cyclohexane is
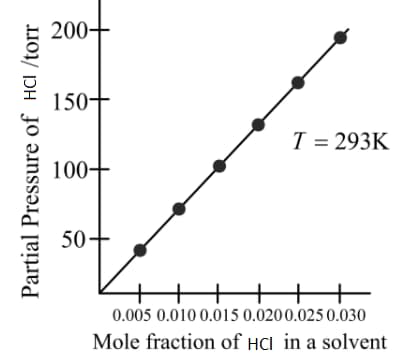
MEDIUM
EASY
EASY

