EASY
Earn 100
Select correct statement(s)
(a)The standard boiling temperature is the temperature at which the vapour pressure of the substance is 1 bar
(b)The normal boiling temperature is the temperature at which the vapour pressure of the substance is 1 atm
(c)Substances for which and are called super critical fluids
(d)All the above are correct statements
100% studentsanswered this correctly
Important Questions on States of Matter
MEDIUM
MEDIUM
MEDIUM
Based on the given figure, the number of correct statement/s is/are
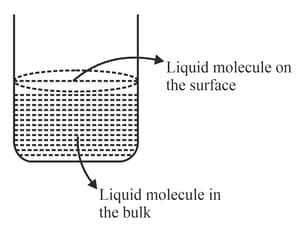
A. Surface tension is the outcome of equal attractive and repulsion forces acting on the liquid molecule in bulk.
B. Surface tension is due to uneven forces acting on the molecules present on the surface.
C. The molecule in the bulk can never come to the liquid surface.
D. The molecules on the surface are responsible for vapour pressure if the system is a closed system.
EASY
EASY
MEDIUM

EASY
HARD
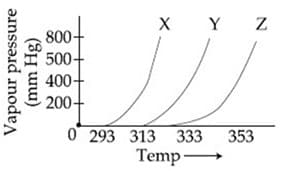
The following inferences are made:
has higher intermolecular interactions compared to
has lower intermolecular interactions compared to
has lower intermolecular interactions compared to
The correct inferences is/are:
MEDIUM
EASY
HARD
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
HARD
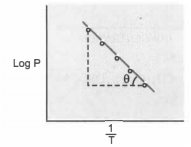
The variation of vapour pressure with temperature for a liquid was studied by plotting versus as shown in the Figure. The slope of the line was found to be . Then latent heat of vaporisation of the given liquid is
MEDIUM

