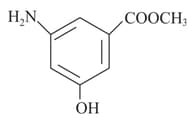
Select the correct match : (consider axis as internuclear axis)
| Column I | Column II |
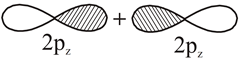 |
No nodal plane in resulting |
| Column I | Column II |
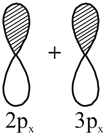 |
Non bonding molecular orbital |
| Column I | Column II |
| orbital of carbon overlap with orbital of oxygen atom forming and |
| Column I | Column II |
| Peroxide ion | bond is present |
Important Questions on Chemical Bonding and Molecular Structure
Given below are two statements : One is labelled as Assertion and the other is labelled as Reason
Assertion : Zero orbital overlap is an out of phase overlap.
Reason : It results due to different orientation/ direction of approach of orbitals.
In the light of the above statements. Choose the correct answer from the options given below
The potential energy curve for formation as a function of internuclear distance of the atoms is shown below.
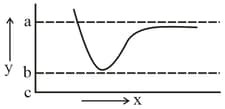
The bond energy of is :
Given below are two statements.
Statement I: The presence of weaker -bonds make alkenes less stable than alkanes
Statement II: The strength of the double bond is greater than that of carbon-carbon single bond.
In the light of the above statements, choose the correct answer from the options : given below.
Consider the following complex ions, P, Q and R.
The correct order of the complex ions, according to their spin-only magnetic moment values (in B.M.) is
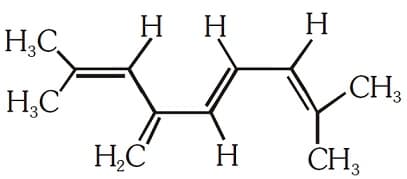
The total number of and bonds present in the following compound are