MEDIUM
Chemistry
IMPORTANT
Earn 100
Select the correct statement(s).
() Polarizability of is lower than
() Polarizability of is higher than
() Polarizability of is lower than
() Polarizability of is higher than
() Polarizability of is higher than
() Polarizability of is lower than
() Polarizability of is higher than
(a)and
(b) and
(c)and
(d)All of these
20% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
Chemistry
IMPORTANT
Which of the following diagram shows the correct change in the ionic character of given compounds according to Fajan's rule?
HARD
Chemistry
IMPORTANT
Stability of and halide complexes are in order :
MEDIUM
Chemistry
IMPORTANT
The correct order of dipole moments of the following compounds is
1.
2.
3.
MEDIUM
Chemistry
IMPORTANT
Identify the set, which contains all the species have permanent dipole moment at room temperature?
HARD
Chemistry
IMPORTANT
The experimental value of the dipole moment of is . The length of the bond is . The percentage ionic character in is:
MEDIUM
Chemistry
IMPORTANT
Which of the following about and molecules is correct?
EASY
Chemistry
IMPORTANT
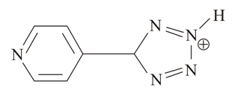
How many lone pairs are present in the given cation?
MEDIUM
Chemistry
IMPORTANT
The geometry around how many carbon atoms is tetrahedral in the given structure?