HARD
Chemistry
IMPORTANT
Earn 100
Select the systematic diagram that represents the correct change in the character in the hybrid orbital of beryllium.
(a)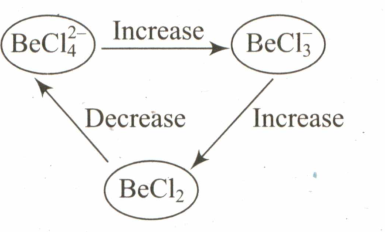

(b)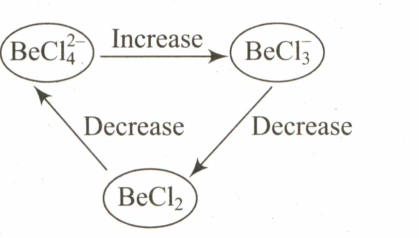

(c)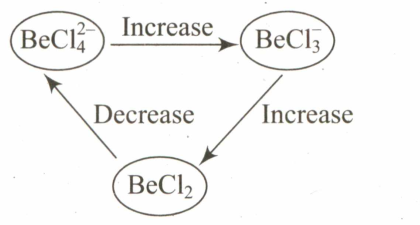

(d)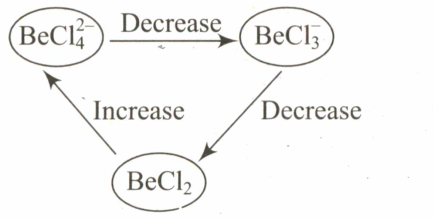

25% studentsanswered this correctly

Important Questions on Chemical Bonding & Molecular Structure
HARD
Chemistry
IMPORTANT
Arrange the following in increasing order of bond angle.
(i) angle in
(ii) angle in
(iii) Adjacent angle in
(iv) angle in
HARD
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
