MEDIUM
Earn 100
Show the coordination number of the metal ion, its oxidation number, the number of electrons in the orbitals and the number of unpaired electrons in orbitals, respectively, in the complex - .
(a)
(b)
(c)
(d)
100% studentsanswered this correctly
Important Questions on Coordination Compounds
EASY
The co-ordination number of and in the complex ions, and are respectively :
EASY
The coordination numbers of and in and respectively are:
(en ethane-1, 2-diamine)
EASY
Which one of the following is an ambidentate ligand?
EASY
The oxidation number of in ion is
EASY
Amongst the following, identify the species with an atom in oxidation state.
HARD
Which among the following coordination compounds does not have coordination number equal to number of ligands?
MEDIUM
Which among the following is a neutral complex?
EASY
Oxidation number and coordination number of silver in Tollen's reagent are respectively:
EASY
Which of the following is an example of homoleptic complex?
MEDIUM
The maximum possible denticities of a ligand given below towards a common transition and inner-transition metal ion, respectively, are:
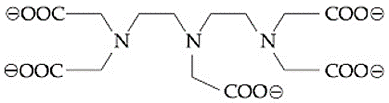
EASY
Which of the following is not an anionic complex?
EASY
Which molecule/ion among the following cannot act as a ligand in complex compounds?
MEDIUM
Molar conductance of a complex of cobalt is zero. Then its structure would be
MEDIUM
The covalency of in is:
MEDIUM
Identify monodentate ligand from following
EASY
Which one of the following metal complexes is most stable?
MEDIUM
The coordination number, oxidation state, number of -electrons and number of unpaired electrons of metal in are respectively
MEDIUM
The following ligand is:
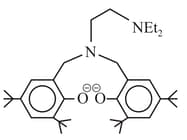
EASY
The correct charge and co-ordination number of in is
EASY
The sum of coordination number and oxidation number of metal in the complex (Where en is ethylenediamine) is:

