EASY
Earn 100
Silicon tetrachloride is prepared by of various silicon compounds such as ferrosilicon, silicon carbide, or mixtures of silicon dioxide and carbon.
50% studentsanswered this correctly
Important Questions on The p-Block Elements
EASY
EASY
(a) is used as a refrigerant for ice cream and frozen food.
(b) The structure of contains twelve six-membered carbon rings and twenty-five membered carbon rings.
(c) , a type of zeolite, is used to convert alcohols into gasoline.
(d) is a colourless and odourless gas.
MEDIUM
MEDIUM
MEDIUM
| Column | Column | ||
| Silica gel | Transistor | ||
| Silicon | Ion-exchanger | ||
| Silicone | Drying agent | ||
| Silicate | Sealant |
MEDIUM
EASY
EASY
EASY
is a monoanion having pyramidal geometry. Both and are neutral compounds. Choose the correct option(s).
EASY
EASY
Tin in state acts as reducing agent while lead in state acts as strong oxidising agent.
Silicon exists as both and forms.
The hybridisation of carbon in fullerene is .
Among and lowest melting point is for .
MEDIUM
MEDIUM
MEDIUM
Identify the correct statements from the following:
a) Silicones are hydrophobic in nature.
b) The repeating unit in silicones is
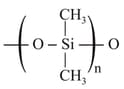
c) In a molecule, twenty rings and twelve rings are present.
d) Dehydration of formic acid by concentrated at gives .
e) The distance between two layers in graphite is .
EASY
EASY
EASY
MEDIUM
EASY

