MEDIUM
Earn 100
Sodium carbonate extract is prepared by:
(a)Fusing soda and mixture and then extracting with water
(b)Dissolving and mixture in dil.
(c)Boiling and mixture in dil.
(d)Boiling and mixture of salt in distilled water
50% studentsanswered this correctly
Important Questions on Organic Chemistry - Some Basic Principles and Techniques
HARD
A white precipitate was formed when was added to water extract of an inorganic salt. Further, a gas with characteristic odour was released when the formed white precipitate was dissolved in dilute HCI. The anion present in the inorganic salt is:
EASY
In the detection of II group acid radical, the salt containing chloride is treated with concentrated sulphuric acid, the colourless gas is liberated. The name of the gas is
MEDIUM
Match List I with List II.
| List-I (Anion) |
List-II (gas evolved on reaction with dil. ) |
||
| (A) | (I) |
Colourless gas which turns lead acetate paper black. |
|
| (B) | (II) |
Colourless gas which turns acidified potassium dichromate solution green. |
|
| (C) | (III) | Brown fumes which turns acidified KI solution containing starch blue. | |
| (D) | (IV) | Colourless gas evolved with brisk effervescence, which turns lime water milky. |
Choose the correct answer from the options given below
HARD
Match the organic compounds in column - with the Lassaigne's test result in column - appropriately:
| Column – | Column - | ||
| A. | Aniline | i. | Red colour with |
| B. | Benzene sulfonic acid | ii. | Violet color with sodium nitroprusside |
| C. | Thiourea | iii. | Blue color with and acidic solution of |
EASY
On heating, lead (ll) nitrate gives a brown gas (A). The gas (A) on cooling changes to a colourless solid/liquid (B). (B) on heating with NO changes to ablue solid (C). The oxidation number of nitrogen in solid (C) is:
EASY
The correct match between item and item is
| Item (Compound) | Item (Reagent) | ||
| Lysine | naphthol | ||
| Furfural | Ninhydrin | ||
| Benzyl alcohol | |||
| Styrene | Ceric ammonium nitrate | ||
MEDIUM
Which combines with to form brown complex?
EASY
Sodium extract is heated with concentrated before testing for halogens because :
EASY
Reaction of an inorganic sulphite with dilute generated compound . Reaction of with gives . Further, the reaction of with and water affords compound and , respectively, are :
MEDIUM
Sodium nitroprusside, when added to an alkaline solution of sulphide ions, produce a
EASY
Acetic acid reacts with sodium metal at room temperature to produce
EASY
When treated with conc. yields gas which further reacts with to generate a white solid reacts with dil. to produce the same gas the solid is
MEDIUM
In the following reaction
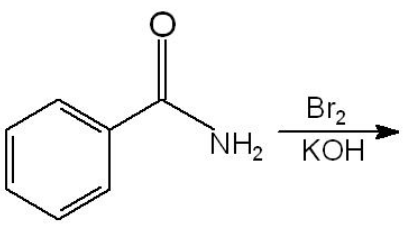
The major product is
MEDIUM
A white sodium salt dissolves readily in water to give a solution which is neutral to litmus. When silver nitrate solution is added to the before mentioned solution, a white precipitate is obtained which does not dissolve in dilute nitric acid. The anion is:
EASY
The reddish brown precipitate formed in the Fehling's test for aldehydes (RCHO) is due to the formation of
MEDIUM
When nitrogen and chlorine containing organic compound is reacted with sodium metal, it forms
EASY
Which of the following compound will give blood red colour while doing the Lassaigne's test for .
HARD
When a mixture of and concentrated is heated in a dry test tube, a red vapour is evolved. This vapour turns an aqueous solution of yellow due to the formation of and respectively, are:
MEDIUM
and both when dissolved in water containing ions the pair of species formed is:
MEDIUM
The sodium salt of an organic acid produces effervescence with concentrated . reacts with the acidified aqueous solution to give a white precipitate which decolourises acidic solution of . is

