EASY
JEE Main
IMPORTANT
Earn 100
Sodium extract is heated with concentrated before testing for halogens because :
(a) and AgCN are soluble in acidic medium.
(b)Silver halides are totally insoluble in nitric acid.
(c) and , if present, are decomposed by conc. and hence do not interfere in the test.
(d)Ag reacts faster with halides in acidic medium
70.37% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
EASY
JEE Main
IMPORTANT
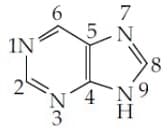
The which contribute least to the basicity of the compound is :
HARD
JEE Main
IMPORTANT
1.4 g of an organic compound was digested according to Kjeldahl's method and the ammonia evolved was absorbed in 60 mL of M/10 solution. The excess sulphuric acid required 20 mL of M/10 NaOH solution for neutralization. The percentage of nitrogen in the compound is:
MEDIUM
JEE Main
IMPORTANT
The optically inactive compound from the following is:
MEDIUM
JEE Main
IMPORTANT
The correct order of basicity is
EASY
JEE Main
IMPORTANT
The potassium ferrocyanide solution gives a Prussian blue colour, when added to :
HARD
JEE Main
IMPORTANT
A sample of an organic compound was digested with conc. and then distilled with . The ammonia gas evolved was passed through of . The used acid required of for complete neutralization. The mass percentage of nitrogen in the organic compound is
HARD
JEE Main
IMPORTANT
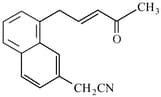
Number of electrophillic centres in the given compound is___
MEDIUM
JEE Main
IMPORTANT
of an organic compound which contains only carbon and hydrogen on complete combustion gives of and of water. The percentage of carbon and hydrogen in the organic compound are respectively
