HARD
Earn 100
Sodium sodium salt of butanoic acid is subjected to decarboxylation in presence of soda lime?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Hydrocarbons
MEDIUM
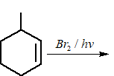
EASY
MEDIUM
Which hydrogen in compound is easily replaceable during bromination reaction in presence of light ?
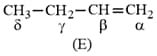
HARD
Isomers of hexane, based on their branching, can be divided into three distinct classes as shown in the figure.
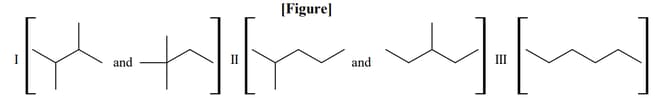
The correct order of their boiling point is
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
EASY
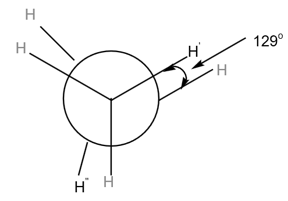
MEDIUM
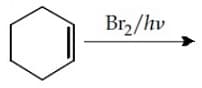
MEDIUM
EASY
HARD
The total number of monohalogenated organic products in the following (including stereoisomers) reaction is
EASY
EASY

