MEDIUM
8th ICSE
IMPORTANT
Earn 100
Solve the following cross-word puzzle.
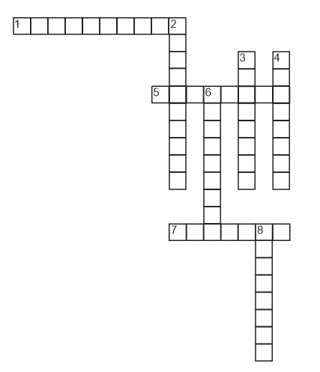
Across
1. Total number of protons and neutrons
5. Subatomic particles with no charge
7. Positively charged subatomic particles
Down
2. He proposed planetary model
3. These elements have same atomic number but different mass number
4. Charge on anode rays
6. Maximum number of electrons in N shell
8. Charge on cathode rays

Across
1. Total number of protons and neutrons
5. Subatomic particles with no charge
Down

Important Questions on Atomic Structure
EASY
8th ICSE
IMPORTANT
EASY
8th ICSE
IMPORTANT
MEDIUM
8th ICSE
IMPORTANT
MEDIUM
8th ICSE
IMPORTANT
MEDIUM
8th ICSE
IMPORTANT
MEDIUM
8th ICSE
IMPORTANT
MEDIUM
8th ICSE
IMPORTANT
MEDIUM
8th ICSE
IMPORTANT
