EASY
10th CBSE
IMPORTANT
Earn 100
Some drops of acetic acid are poured over a pH paper. The colour produced on the paper will be
(a)Green.
(b)Violet.
(c)Blue.
(d)Orange.
50% studentsanswered this correctly

Important Questions on Acids, Bases and Salts
EASY
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
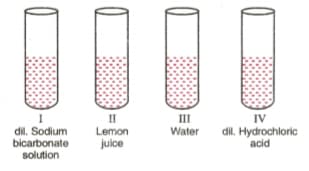
A student was provided with four samples of solutions as shown in figures (I), (Il), (Ill), and (IV). He determined pH value of each solution by using pH paper. The correct sequence of colour change of pH paper observed by the student will be:
EASY
10th CBSE
IMPORTANT
