EASY
12th ICSE
IMPORTANT
Earn 100
Some energy levels of a molecule are shown in the figure. The ratio of the wavelengths is given by :
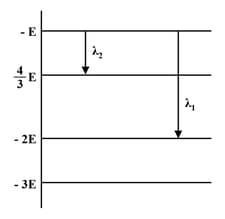

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Atom, Origin of Spectra : Bohr's Theory of Hydrogen Atom
HARD
12th ICSE
IMPORTANT
The radiation corresponding to transition of hydrogen atom falls on a metal surface to produce photoelectrons. These electrons are made to enter a magnetic field of . If the radius of the largest circular path followed by these electrons is 10.0mm, the work function of the metal is close to
EASY
12th ICSE
IMPORTANT
The ratio of wavelength of the last line of Balmer series and the last line Lyman series is:
EASY
12th ICSE
IMPORTANT
The wavelength of the first spectral line in the Balmer series of hydrogen atom is .The wavelength of the second spectral line in the Balmer series of singly-ionized helium atom is:
EASY
12th ICSE
IMPORTANT
If an electron in a hydrogen atom jumps from the orbit to the orbit, it emits a photon of wavelength . When it jumps from the orbit to the orbit, the corresponding wavelength of the photon will be
EASY
12th ICSE
IMPORTANT
Given the value of Rydberg's constant is , the wave number of the last line of the Balmer's series in hydrogen spectrum will be:
EASY
12th ICSE
IMPORTANT
The largest wavelength in the ultraviolet region of the hydrogen spectrum is . The smallest wavelength in the infrared region of the hydrogen spectrum (to the nearest integer) is:
EASY
12th ICSE
IMPORTANT
The ratio of kinetic energy to the total energy of an electron in a Bohr orbit of the hydrogen atom, is?
MEDIUM
12th ICSE
IMPORTANT
If the series limit frequency of the Lyman series is , then the series limit frequency of the Pfund series is :
