Some solutions are given in the table below. Identify the states of the solute and the solvent in each of them and complete the table.
Solution-Constituents
State of the solute
State of the solvent
State of the solution
Brass(Zinc + Copper)
Solid
Solid
_____
Salt solution(Salt+ water)
_____
_____
Liquid
Soda water(Carbon dioxide + water)
Gaseous
_____
_____
A mixture of alcohol and water
Liquid
_____
_____
Is there any relation between the state of the solution and that of the solvent?

Important Questions on Solutions
Take equal amounts of water in two glass tumblers. Add one or two crystals of potassium permanganate in the first tumbler and four or five crystals in the second and stir. Observe the difference in the colour of the solutions in the two tumblers. What is the reason for the difference in the colour?
Do different materials dissolve to the same extent in a given solvent?
While preparing saturated solutions of various solutes in a definite amount of a given solvent under the same conditions, will the amount of solutes getting dissolved be the same? Try to find out.
Given is a graph that connects the solubility and the temperature of certain salts.
Examine the graph and find out the following:
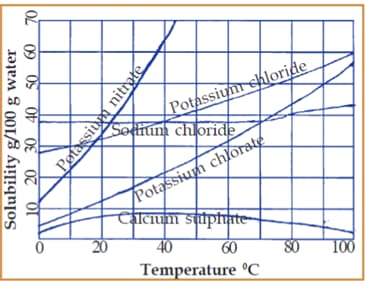
Which substance shows the maximum increase in solubility as temperature increases?
Given is a graph that connects the solubility and the temperature of certain salts.
Examine the graph and find out the following:
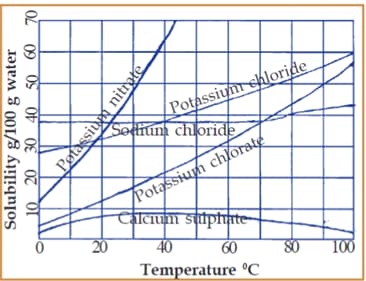
Which salts have the same solubility at a temperature of ?
