Some water in a glass beaker is heated from below, as shown in the diagram.
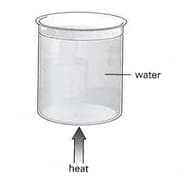
Name the process by which thermal energy is transferred
Throughout the water.


Important Questions on Thermal (Heat) Energy Transfers
Some water in a glass beaker is heated from below, as shown in the diagram.
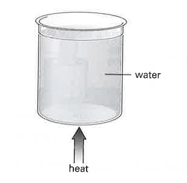
As thermal energy is supplied, the temperature of the water begins to rise. Although the supply of energy remains constant, eventually the temperature becomes steady at . Suggest why this happens.
Some water in a glass beaker is heated from below, as shown in the diagram.
.png)
The rate of energy supply is increased. The temperature of the water begins to rise again, but eventually becomes steady at a higher temperature. This time many bubbles are seen throughout the water.
State what is now happening to the water.
Some water in a glass beaker is heated from below, as shown in the diagram.
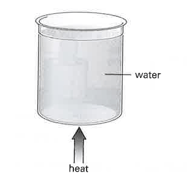
The rate of energy supply is increased. The temperature of the water begins to rise again, but eventually becomes steady at a higher temperature. This time many bubbles are seen throughout the water.
What gas do the bubbles contain Choose one from
air, hydrogen, oxygen, steam
An iron rod and a copper rod of equal length are each held by hand at one end, with the other end in the flame from a Bunsen burner, as shown in the diagram.
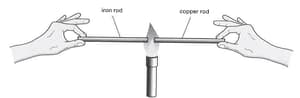
The copper rod becomes too hot much sooner than the iron rod. What does this information tell you about iron and copper
Gas has to be above a certain temperature before it burns.The diagram shows two similar wire gauzes, one made of iron wire and made of copper wire. Each is held over a Bunsen burner. When the gas supply is turned on and ignited the gauze, the effect is as shown in the diagram.
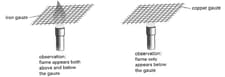
How can these observations be explained
