MEDIUM
Earn 100
Spectral lines for -atom were observed as shown below where n1 to n5 are successive shells. If belongs to visible region, then which of the following statements are correct for the transition?
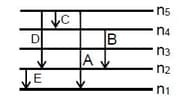
(A) There can be a line in region
(B) and lines belongs to Infrared region.
(C) Line having shortest wavelength is
(D) Line having least energy is

(B) and lines belongs to Infrared region.
(C) Line having shortest wavelength is
(D) Line having least energy is
(a) and
(b) and
(c) and
(d) and
50% studentsanswered this correctly
Important Questions on Atomic Physics
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
EASY
HARD
EASY
MEDIUM
MEDIUM
HARD
EASY
HARD
EASY
EASY
EASY

