MEDIUM
JEE Main
IMPORTANT
Earn 100
Spin only magnetic moment of an octahedral complex of in the presence of a strong field ligand in BM is:
(a)
(b)
(c)
(d)
62.5% studentsanswered this correctly

Important Questions on Coordination Compounds
MEDIUM
JEE Main
IMPORTANT
Which one of the following species doesn't have a magnetic moment of (spin only value)?
EASY
JEE Main
IMPORTANT
An aqueous solution of was heated with excess sodium cyanide in presence of strong oxidizing agent to form The total change in number of unpaired electrons on metal centre is ___________.
MEDIUM
JEE Main
IMPORTANT
The correct order of intensity of colors of the compounds is:
HARD
JEE Main
IMPORTANT
Simplified absorption spectra of three complexes ((i) and (ii) and (iii)) of ion are provided below; their values are marked as and respectively. The correct match between the complexes and their values is:
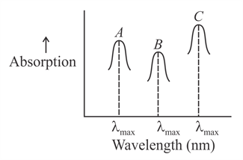
(i)
(ii)
(iii)
EASY
JEE Main
IMPORTANT
The one that is not expected to show isomerism is:
HARD
JEE Main
IMPORTANT
Consider that metal ion forms a complex with aqua ligands, and the spin only magnetic moment of the complex is . The geometry and the crystal field stabilization energy of the complex is :
MEDIUM
JEE Main
IMPORTANT
For octahedral and tetrahedral complexes, consider the following statements :
both the complexes can be high spin.
complex can very rarely be of low spin.
with strong field ligands, complexes can be low spin.
aqueous solution of is yellow in color.
The correct statements are :
MEDIUM
JEE Main
IMPORTANT
The oxidation states of iron atoms in compounds , and , respectively, are and . Then sum of and is ...........
