HARD
Earn 100
State any two limitations of Crystal Field Theory.
Important Questions on Coordination Compounds
MEDIUM
EASY
MEDIUM
MEDIUM
HARD
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
(Atomic No. = Ti : 22, Cr : 24 and Mo : 42)
MEDIUM
HARD
MEDIUM
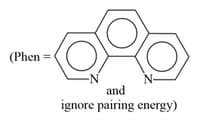
EASY
MEDIUM
MEDIUM
HARD

