EASY
10th ICSE
IMPORTANT
Earn 100
State the effect of increase of pressure on the melting point of ice.

Important Questions on Calorimetry
EASY
10th ICSE
IMPORTANT
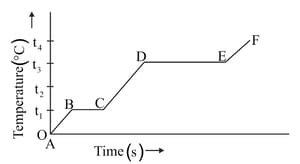
(a) What do the parts AB, BC, CD and DE represent?
(b) What is the melting point of the substance?
(c) What is the boiling point of the substance?
EASY
10th ICSE
IMPORTANT
The diagram in figure below shows the change of phases of a substance on a temperature-time graph.
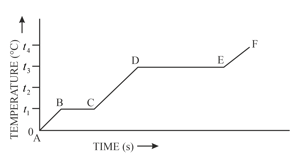
What do the parts and represent ?
EASY
10th ICSE
IMPORTANT
The diagram in figure below shows the change of phases of a substance on a temperature-time graph.
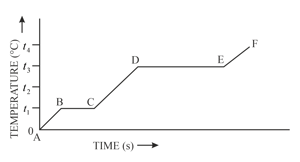
What is the melting point of the substance?
EASY
10th ICSE
IMPORTANT
The diagram in figure below shows the change of phases of a substance on a temperature-time graph.
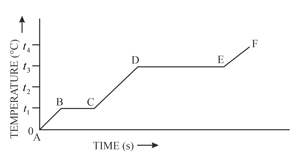
What is the boiling point of the substance?
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
