MEDIUM
Earn 100
State the formula to calculate the percentage purity of pure substances.
Important Questions on Reacting Masses and Chemical Equations
HARD
With the help of an activity prove the law of conservation of mass.
EASY
In a compound, the constituent elements are present in a _____.
HARD
State and explain the law of constant proportions by giving an example.
EASY
Electrolysis of water is a decomposition. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is;
EASY
State the law of conservation of mass.
MEDIUM
How will you show that there is no change in mass during a chemical reaction?
MEDIUM
Who formulated the law of constant composition?
MEDIUM
Law of conservation of mass holds true and can be verified in a school laboratory
MEDIUM
In a reaction, of calcium carbonate on heating gave of calcium oxide and of carbon dioxide. Show that these observations are in agreement with the law of conservation of mass.
HARD
Find the percentage composition of sodium sulphate ().
HARD
When of carbon is burnt in oxygen, of carbon dioxide is produced. What mass of carbon dioxide will be formed when of carbon is burnt in of oxygen? Which law of chemical combination will govern your answer?
MEDIUM
1.31 g of zinc on heating in air gave 1.63 g of white zinc oxide. 2.65 g of zinc oxide on reduction with hydrogen gave 2.12 g of zinc. Show that these figures are in accordance with the law of constant proportions.
EASY
State law of conservation of mass.
MEDIUM
Explain law of constant proportion.
EASY
State the law of constant proportions.
MEDIUM
How will you calculate mass percentage of an element?
MEDIUM
Select from the following figure(s) that correctly represent(s) the experimental set-up for the verification of conservation of mass in a chemical reaction.
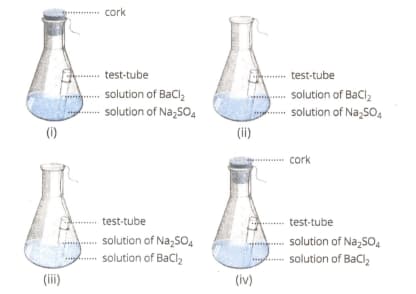
EASY
State the law of conservation of mass.
MEDIUM
State law of constant proportions.
HARD
A sample of compound of oxygen and boron was found by analysis to contain of boron and of oxygen. Calculate the percentage composition of the compound by weight.

