EASY
10th CBSE
IMPORTANT
Earn 100
State the modern periodic law.

Important Questions on Periodic Classification of Elements
MEDIUM
10th CBSE
IMPORTANT
What is a period in a periodic table? How does the electronic configuration change in a period with increase in atomic number from left to right?
MEDIUM
10th CBSE
IMPORTANT
Discuss two advantages of the periodic table.
HARD
10th CBSE
IMPORTANT
Magnesium forms the following compounds:
Magnesium oxide — MgO
Magnesium hydroxide — Mg(OH)2
Magnesium sulphate — MgSO4
If radium too belongs to the same group as magnesium, what would be the formula of radium oxide, radium hydroxide and radium sulphate?
HARD
10th CBSE
IMPORTANT
For the main groups of the periodic table, the metallic properties of the elements vary approximately with their position as shown in the chart below
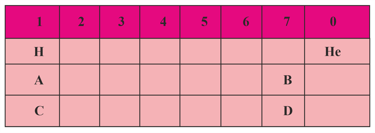
(a) Where will you find the most metallic element?
(b) Where will you find the most nonmetallic element?
(c) Where will you find the smallest atom?
MEDIUM
10th CBSE
IMPORTANT
Name two other elements which are in the same family as
(a) carbon
(b) fluorine and
(c) sodium.
MEDIUM
10th CBSE
IMPORTANT
Write the names of first four members of the halogen family. Write their symbols in the order of increasing atomic number. How are their melting points expected to be related to their atomic numbers?
MEDIUM
10th CBSE
IMPORTANT
From their positions in the periodic table, select the atom with the larger size in each of the following pairs:
(a) Li and Be
(b) Na and Mg
(c) Cl and I
(d) B and C
MEDIUM
10th CBSE
IMPORTANT
In what respect does modern statement of the periodic law differ from that stated in Mendeleev’s periodic law?
